Pharma Supply Chain Transparency & Digital Asset
Blockchain transforms pharmaceutical transparency by automating track-and-trace via Hyperledger Fabric. Integrating IoT sensors, this platform eliminated supply chain visibility gaps, driving 100% counterfeit detection and a 75% reduction in compliance costs through real-time administrative efficiency



Problem Statement
Manufacturers lack a streamlined platform to track medications, verify distributor legitimacy, manage batch recalls, and monitor cold-chain compliance, increasing overall pharmaceutical supply chain risks.
Goals
Automate product tracking and stakeholder coordination.
Secure communication with immutable records and clinical updates.
Integrate IoT sensors and administrative audit tools
Enhance efficiency by blockchain smart contract workflows.

Product Overview
The Blockchain Supply Chain Platform is a unified network for stakeholders, providing everything required to manage pharmaceutical tracking and safety. Users can verify product authenticity, monitor temperature excursions, review digital health credentials, and access audit logs.
The system also offers IoT syncing, automated alerts, and recall triggers for a secure experience including immutable records and advanced data privacy. With smart contracts, real-time verification, and integration options for manufacturers, the platform ensures every shipment is compliant and effortless. Whether it’s managing batch recalls, navigating regulatory audits, or enjoying seamless data portability, this blockchain is built to maximize patient safety and transparency.
Responsibility
Tools
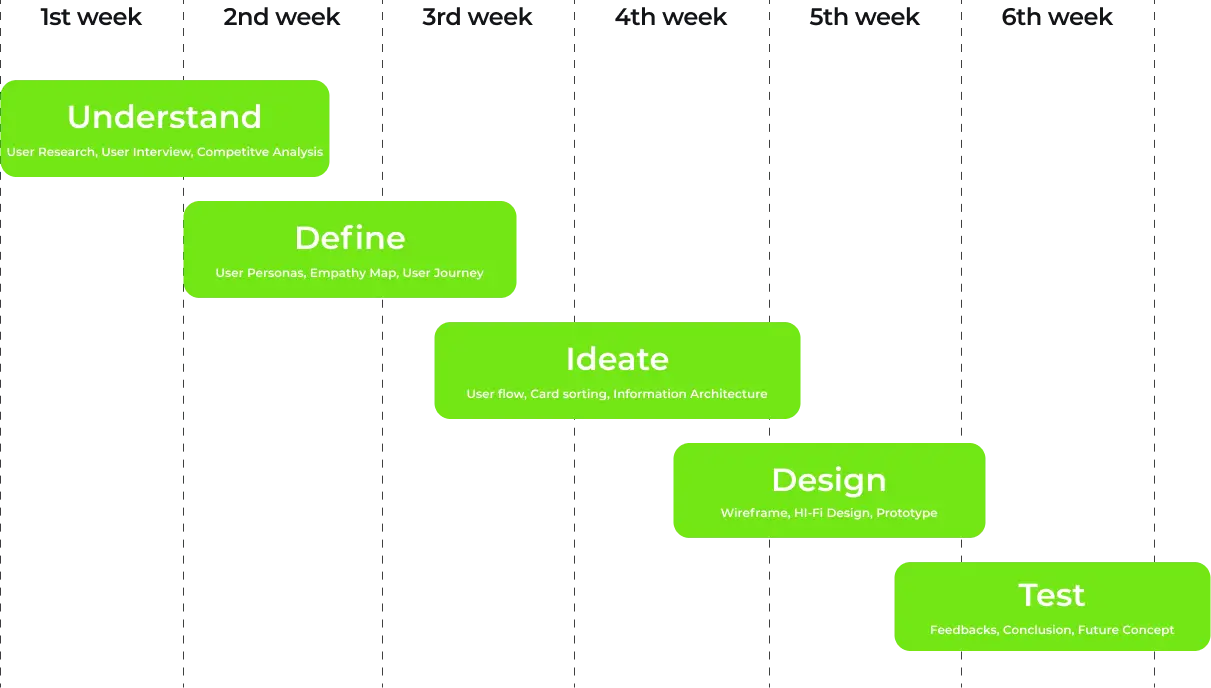
Design Process
The design process follows a structured approach: Understand user needs, define key features, ideate solutions, design the interface, and test for usability and performance. This ensures a user-centered, functional, and engaging app experience.

Understand
User Research
User Interview
Competitive Analysis

Define
User Personas
Empathy Map
User Journey

Ideate
Information Architecture

Design
Wireframe
Hi-FI Design
Prototype

Test
Feedbacks
Conclusion
Future Concept
Design Timeline
Target Audience
Patients requiring authentic medications and portable health credentials.
Healthcare providers seeking supply chain transparency and compliance efficiency.
Stakeholders interested in pharmaceutical tracking, cold-chain safety, and regulatory audit readiness.
Industry participants looking for a decentralized and secure platform for pharmaceutical logistics.





User Research
User research involved audits and interviews with pharmaceutical manufacturers and distributors, revealing a need for a transparent platform. Users prioritized product authenticity, cold-chain visibility, and rapid recall execution. Automated triggers and real-time monitoring were key desires for enhancing supply chain integrity.
Competitive Analysis
Current supply chain tools lack a comprehensive platform integrating blockchain, real-time IoT sensors, and immutable records. Competitors offer limited tracking features, missing the opportunity for instant counterfeit detection, automated audit reporting, and a seamless regulatory experience.
Service Name
ERP System
Track-and-Trace
Manual Ledger
Blockchain Hub
Service Info
Legacy enterprise resource planning
Standard medication tracking
Manual paper-based records
Permissioned blockchain network
Prediction




Syncing




Alerts




Management




AI Assistant




Factory Info




Automation




Key Features
Blockchain-powered ledger provides 24/7 immutable tracking support and personalized data ownership.
Direct IoT syncing increases operational speed with real-time readings and instant alerts.
Allows stakeholders to access medication history and seamless updates on product authenticity.
Smart contracts unlock administrative savings and rapid recalls, reducing manual verification tasks.
Quantitative Research
The Blockchain Supply Chain & Digital Asset project aims to provide a secure and transparent experience for healthcare stakeholders and patients. Our quantitative research involved analyzing pharmaceutical logistics data, focusing on functionalities like track-and-trace, cold-chain monitoring, audit automation, and patient data portability.
Screeners
Stakeholders seeking a permissioned blockchain interface for real-time pharmaceutical tracking activities.
- Manufacturers interested in managing shipment pipelines and subscribing for automated temperature alerts.
- Distributors looking for verified product authenticity templates and global supply chain availability.
- Regulators who value instant audit response times and automated compliance reporting support.
- Patients aged 18-80 regularly engaging with digital health platforms for prescriptions and records.
Key Performance (Baseline vs. Target)
2-4 Weeks to <48 Hours
Audit Readiness Time: Time from initial request to a ready-to-audit environment reduced via automation.
2-4 Weeks to <4 Hours
Recall Execution Lead: Drastic reduction in the time required for contaminated doses to move from detection to quarantine.
$300M to $50M
Compliance Spend Reduction: Percentage of pharma budget spent on routine manual reporting and audits versus strategic safety.
Monthly to Instant
Verification Frequency: Shifted from manual batch reconciliation to a real-time production unit verification cadence.
24+ Hours to <15 Mins
Time to Remediate Excursion: Time from deviation detection to resolution through automated sensor monitoring and alerts.
7.3x ROI
Overall Operational Savings: Reduction in total supply chain spend through anti-counterfeiting, automation, and efficiency.
1. Fragmented Supply Chain Systems
Stakeholders currently have to rely on multiple channels to access drug availability, audit logs, temperature records, and shipment updates. This fragmented access to information creates confusion, delays, and unnecessary time spent searching for accurate pharmaceutical supply chain guidance.
2. Lack of Real-time Visibility
Existing tracking methods do not provide tailored information based on individual batch history, cold-chain needs, or urgent recall requirements. Important updates, alerts, or storage instructions are often generic and not customized to each shipment’s specific medical situation.
3. Absence of Patient Control
There is a lack of structured digital tools or automated systems that recognize patient urgency. Patients want clearer advantages from manufacturer participation, such as exclusive health resources, 24/7 authenticity access, or digital record services that add tangible convenience.

User Persona: David Chen

Name:
Marcus Chen
Age:
42
EDUCATION:
Senior Cloud Architect
Job:
Lead Platform Engineer
Location:
Seattle, USA
HOBBIES:
Reviewing system breakthroughs, family time, travel
Bio
David is a regional supply chain director and an essential member of a global distribution network committed to operational excellence. He manages high-volume shipments across various hubs and facilities, necessitating that he stay informed regarding serialization laws, storage protocols, and inventory updates.
Personality
Responsible
Analytical
Compliance-driven
Efficient
Pain Points
Difficulty staying updated with manual reconciliation and fragmented distribution reports.
Struggles to verify product legitimacy across different unauthorized market operators.
Desires more direct, transparent, and faster temperature alerts from IoT sensor networks.
Goal
Maintain real-time awareness of cold-chain compliance and professional safety standards.
Improve audit readiness through enhanced blockchain transparency and immutable data clarity.
Rely on automated track-and-trace for distribution representation and inventory protection.
User Persona: Sarah Jenkins

Name:
Sarah Jenkins
Age:
48
EDUCATION:
Global Logistics Management
Job:
Senior Logistics Manager
Location:
London, UK
HOBBIES:
Technical writing, sailing with family, volunteering
Bio
Sarah is a senior logistics manager at a major pharmaceutical firm committed to global drug safety. She oversees complex distribution across 60+ countries, requiring her to stay informed about unit tracking, cold-chain compliance, and global regulatory updates.
Personality
Organized
Detail-oriented
Safety-focused
Strategic
Pain Points
Difficulty tracking real-time visibility across 40+ global distribution hubs.
Struggles to verify product legitimacy across different unauthorized market operators.
Struggles to access consolidated temperature data across different shipping routes.
Goal
Achieve 100% real-time tracking from manufacture to the final patient.
Improve recall efficiency through automated blockchain-driven smart contract triggers.
Rely on digital credentials for seamless product authentication and patient protection.
User Journey Map
Persona: Carlos Martínez (Senior Risk Operations Manager)
Actions
Verification
Collaboration
Compliance
Optimization
Task List
Checks product authenticity in the region.
Shares logistics data with local hospitals.
Prepares documentation for FDA/EU audits.
Analyzes ROI and supply chain efficiency.
Feeling
"Is this batch verified for safe distribution?"
"Everyone needs access to the same ledger."
"The audit trail must be 100% accurate."
"We’ve reduced compliance costs significantly."
Thoughts
Cautious about unauthorized market units.
Collaborative and ready to share data.
Weeks spent on manual reconciliations.
High overhead for routine safety reporting.
Improvement Opportunities
High volume of counterfeit pharmaceutical units.
Siloed data preventing patient portability.
Use smart contracts for automated auditing.
Scale the pilot to all global product lines.
Actions : Action 1
Actions
Checks product authenticity in the region.
Thinking
"Is this batch verified for safe distribution?"
Feeling
Cautious about unauthorized market units.
Pain Point
High volume of counterfeit pharmaceutical units.
Opportunity
QR codes for instant unit authentication.
Actions : Action 2
Actions
Track urgent repair bookings
Thinking
Satisfied with live updates
Feeling
It’s great to see live machine queue updates.
Pain Point
Provide an asset filter for schedules Personalize plant flows for staff
Opportunity
QR codes for instant unit authentication.
Actions : Action 3
Actions
Checks product authenticity in the region.
Thinking
Cautious about unauthorized market units.
Feeling
Cautious about unauthorized market units.
Pain Point
High volume of counterfeit pharmaceutical units.
Opportunity
QR codes for instant unit authentication.
Actions : Action 4
Actions
"Is this batch verified for safe distribution?"
Thinking
"Is this batch verified for safe distribution?"
Feeling
Cautious about unauthorized market units.
Pain Point
High volume of counterfeit pharmaceutical units.
Opportunity
QR codes for instant unit authentication.
User Journey Map
Persona: Sarah Jenkins (Cloud Operations Director)
Actions
Verification
Collaboration
Compliance
Optimization
Task List
Conducts audits on onboarding processes.
How can we enhance team synergy?
Ensures all products meet regulatory standards.
Implements continuous improvement strategies.
Feeling
Are we capturing all necessary data points?
How can we enhance team synergy?
What are the most recent compliance updates?
How can we streamline our operations?
Thoughts
Cautiously optimistic about the results.
Energized by collaborative efforts.
Anxious about upcoming inspections.
Motivated to maximize efficiency.
Improvement Opportunities
Inconsistent verification protocols.
Limited engagement from some partners.
Complicated compliance documentation.
Inefficiencies in resource allocation.
Actions : Action 1
Actions
Conducts audits on onboarding processes.
Thinking
Are we capturing all necessary data points?
Feeling
Cautiously optimistic about the results.
Pain Point
Inconsistent verification protocols.
Actions : Action 2
Actions
Facilitates regular stakeholder meetings.
Thinking
How can we enhance team synergy?
Feeling
Energized by collaborative efforts.
Pain Point
Limited engagement from some partners.
Actions : Action 3
Task List
Access unit repair records
Feeling
Happy about data accuracy
Thoughts
This automation will be perfect for my staff!
Improvement Opportunities
More integrations for industrial items Introduce industrial resource packs
Actions : Action 4
Task List
Subscribe to AI health alerts
Feeling
Relieved to manage technical content.
Thoughts
The AI should help me stay organized.
Improvement Opportunities
Simplify automated management Enhance plant loyalty benefits
Key Takeaways
The Blockchain Supply Chain Platform is engineered to centralize pharmaceutical operations by offering real-time tracking, cold-chain monitoring, and recall automation within one ecosystem. Our research highlights the necessity for integrated transparency, addressing the frustrations manufacturers face with current manual-based solutions. Features like the IoT syncing, smart contracts, and immutable ledgers add value to the business journey. Results revealed 100% counterfeit detection, while a 75% reduction was achieved for compliance costs and automated audit updates. The platform caters to diverse personas—logistics managers, supply directors, and regulatory stakeholders—ensuring safety and engagement. This project redefines how medical institutions connect with their global distribution networks, enhancing both security and loyalty.

